Cell Division & Developmental Growth Programs
Understanding the Molecular Mechanism of Cell Division
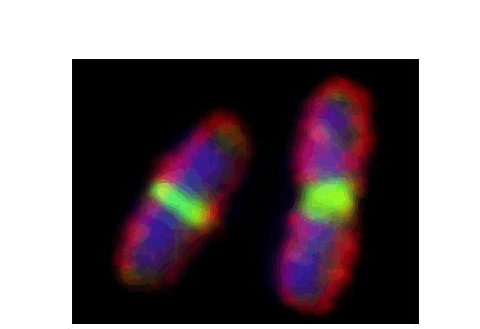
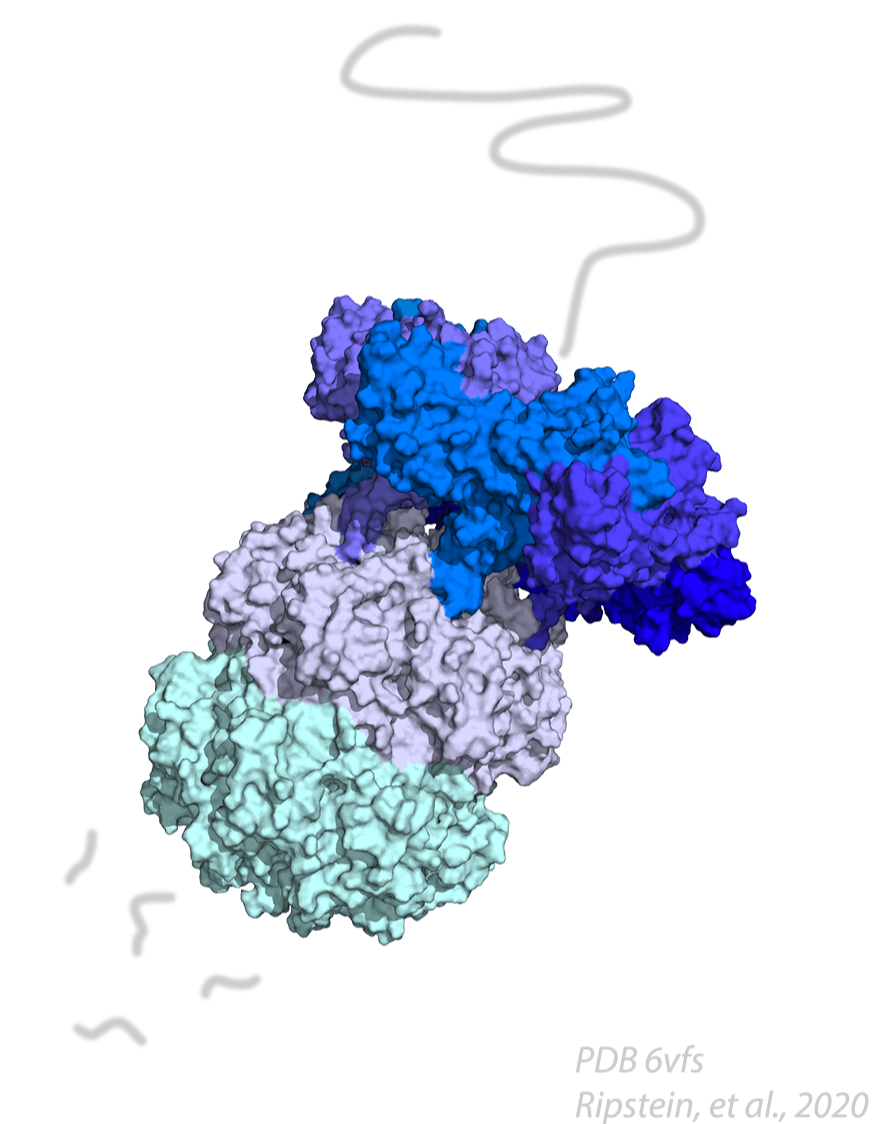
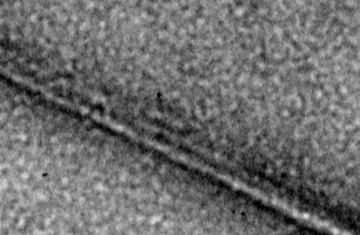
FtsZ is a highly conserved bacterial cell division protein that is a structural homolog of tubulin and polymerizes to form a dynamic protein structure called the Z-ring at midcell. At the Z-ring, the center of an E. coli cell constricts and new cell wall is built at the septum to divide the cell into two identical progeny cells. Using live cell tracking in vivo and reconstituted division machinery in vitro, we investigate proteins that recruit, stabilize and destabilize the Z-ring, as well as systems responsible for precise timing and placement of the Z-ring in vivo. The actin-like ATPase FtsA polymerizes, coassembles with FtsZ polymers, and together they form the nascent Z-ring at the site of division. We are pioneering methods to study FtsA-FtsZ complexes and determine how they contribute to initiating cell wall synthesis during division.